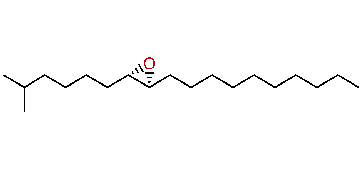
| (7S,8R)-cis-7,8-Epoxy-2-methyloctadecane |
| Formula: | C19H38O |
| CAS#: | 42991-03-7 |
| MW: | 282.51 |
[MS]
[ NMR ]
[Behavioural function]
|
|
Reference(s) for synthesis of (7S,8R)-cis-7,8-Epoxy-2-methyloctadecane [7S8R-disparlure]
| Brown, H.C., and Basavaiah, D. 1983. Pheromone synthesis via organoboranes: a convenient stereospecific synthesis of racemic disparlure, the sex pheromone of the gypsy moth (Porthetria dispar L.). Synthesis. 4:283-284. |
| |
| Bykov, V.I., Goletiani, A.R., Butenko, T.A., and Finkelshtein, E.Sh. 2002. Stereoselective cometathesis of 1,5-cyclooctadiene with ethylene as an efficient pathway to Z-ene biologically active natural products. Dokl. Chem. (Engl. Transl.). 384:163-166. |
| |
| Chan, T.H., and Koumaglo, K. 1985. Silicon in organic synthesis. Stereoselective synthesis of some insect sex pheromones. J. Organomet. Chem. 285:109-120. |
| |
| Friesen, R.W., and Giroux, A. 1994. Can. J. Chem. 72:1857-1865. |
| |
| Jonas, J., Slanina, P., Humpa, O., and Mazal, C. 1988. Collect. Czech. Chem. Comm. 53:857-859. |
| |
| Kel'in, A.V., and Kulinkovich, O.G. 1998. New synthesis of (±)-cis-disparlure, pheromone of female gypsy moth (Porthetria dispar) species. Russ. J. Org. Chem. 34:1446-1448. |
| |
| Koumaglo, K., and Chan, T.H. 1984. Regioselection in the alkylation of trimethylsilylallyl anion - stereoselective synthesis of disubstituted alkenes. Tetrahedron Lett. 25:717-720. |
| |
| Koumbis, A.E., and Chronopoulos, D.D. 2005. A short and efficient synthesis of (+)-disparlure and its enantiomer. Tetrahedron Lett. 46:4353-4355. |
| |
| Marshall, J.A., Jablonowski, J.A., and Jiang, H. 1999. Total synthesis of the gypsy moth pheromones (+)- and (-)-disparlure from a single nonracemic alpha-silyloxy allylic stannane. J. Org. Chem. 64:2152-2154. |
| |
| Prestwich, G.D., Mc Graham, S., Kuo, J.-W., and Vogt, R.G. 1989. Tritium-labeled enantiomers of disparlure. Synthesis and in vitro metabolism. J. Am. Chem. Soc. 111:636-642. |
| |
| Sonnet, P.E. 1980. Olefin inversion. 3. Preparations and reductions of vic-halohydrin trifluoroacetates. J. Org. Chem. 45:154-157. |
| |
| Vig, O.P., Sharma, M.L., Kumari, S., and Vohra, N. 1985. A new synthesis of (Z)-7,8-epoxy-2-methyloctadecane (disparlure). Indian J. Chem. Sect. B. 24:860-861. |
| |
| Wang, K.K., and Chu, K.-H. 1984. Preparation of (Z)-alkenes, ketones and alkynes via trialkyltin chloride induced intramolecular transfer reaction of lithium 1-alkynyltrialkylborates. Stereoselective synthesis of the sex pheromones of the Douglas fir tussock moth, the gypsy moth, and the wild silkmoth Antheraea polyphemus. J. Org. Chem. 49:5175-5178. |
| |
|







