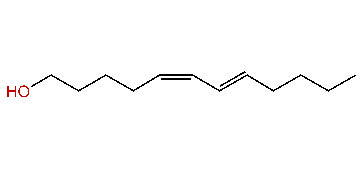
| (Z,E)-5,7-Dodecadien-1-ol |
| Formula: | C12H22O |
| CAS#: | 73416-71-4 |
| MW: | 182.31 |
[MS]
[ NMR ]
[ Kovats ]
[Behavioural function]
|
|
Reference(s) for synthesis of (Z,E)-5,7-Dodecadien-1-ol [Z5E7-12OH]
| Ando, T., Vu, M.H., Yoshida, S., and Takahashi, N. 1982. Stereoselective synthesis of some isomers of dodecadien-1-ol: compounds related to the pine moth sex pheromone. Agric. Biol. Chem. 46:7177. |
| |
| Bestmann, H.J., Koschatzky, K.H., Platz, H., Suess, J., Vostrowsky, O., Knauf, W., Burghardt, G., and Schneider, I. 1982. Pheromone XL. Stereoselektive synthese des pheromonkomplexes von Lasiocampidae-Arten (Lepidoptera); ein sexuallockstoff fur den kiefernspinner Dendrolimus pini. Liebigs Ann. Chem. 7:1359-1365. |
| |
| Brown, H.C., Bhat, N.G., and Basavaiah, D. 1986. Pheromone synthesis via organoboranes: a stereoselective synthesis of (E)-1,3-alkenynes via thexylchloroborane-dimethyl sulfide. Synthesis. 8:674-676. |
| |
| Chisholm, M.D., Steck, W.F., Bailey, B.K., and Underhill, E.W. 1981. Synthesis of sex pheromone components of the forest tent caterpillar, Malacosoma disstria (Huebner) and of the Western tent caterpillar, Malacosoma californicum (Packard). J. Chem. Ecol. 7:159-164. |
| |
| Huang, Y.-Z., Shi, L., and Yang, J. 1987. Highly stereoselective synthesis of Z,E conjugated diene type sex pheromones. J. Org. Chem. 52:3558-3560. |
| |
| Khrimian, A., Klun, J.A., Hijji, Y., Baranchikov, Y.N., Pet'ko, V.M., Mastro, V.C., and Kramer, M.H. 2002. Syntheses of (Z,E)-5,7-dodecadienol and (E,Z)-10,12-hexadecadienol, Lepidoptera pheromone components, via zinc reduction of enyne precursors. Test of pheromone efficacy against the Siberian moth. J. Agric. Food Chem. 50:6366-6370. |
| |
| Stille, J.K., and Groh, B.L. 1987. Stereospecific cross-coupling of vinyl halides with vinyl tin reagents catalyzed by palladium. J. Am. Chem. Soc. 109:813-817. |
| |
| Stille, J.K., and Simpson, J.H. 1987. Stereospecific palladium-catalyzed coupling reactions of vinyl iodides with acetylenic tin reagents. J. Am. Chem. Soc. 109:2138-2152. |
| |
| Trost, B.M., and Martin, S.J. 1984. Alkynyl sulfenylation. A direct approach for nucleophilic addition and substitution of olefins by carbanions. J. Am. Chem. Soc. 106:4263-4265. |
| |
| Xian-Zuo, M. and Huai-Min, W. 1983. Synthesis and field experiment of sex attractant for pine caterpillar, Dendrolimus tabulaeformis. Linye Kexue. 19:137. |
| |
|







